Lowering Barriers to Addiction Care: Sonara’s 2024 Year in Review
2024 has been a transformative year for Sonara Health. Through new opioid treatment program partnerships, clinical studies, and advocacy efforts, we have had more opportunities than ever before to expand access to methadone.
Here’s a closer look at the work we’ve done in 2024 to advance treatment for opioid use disorder:
Increasing Access to Take-Home Methadone
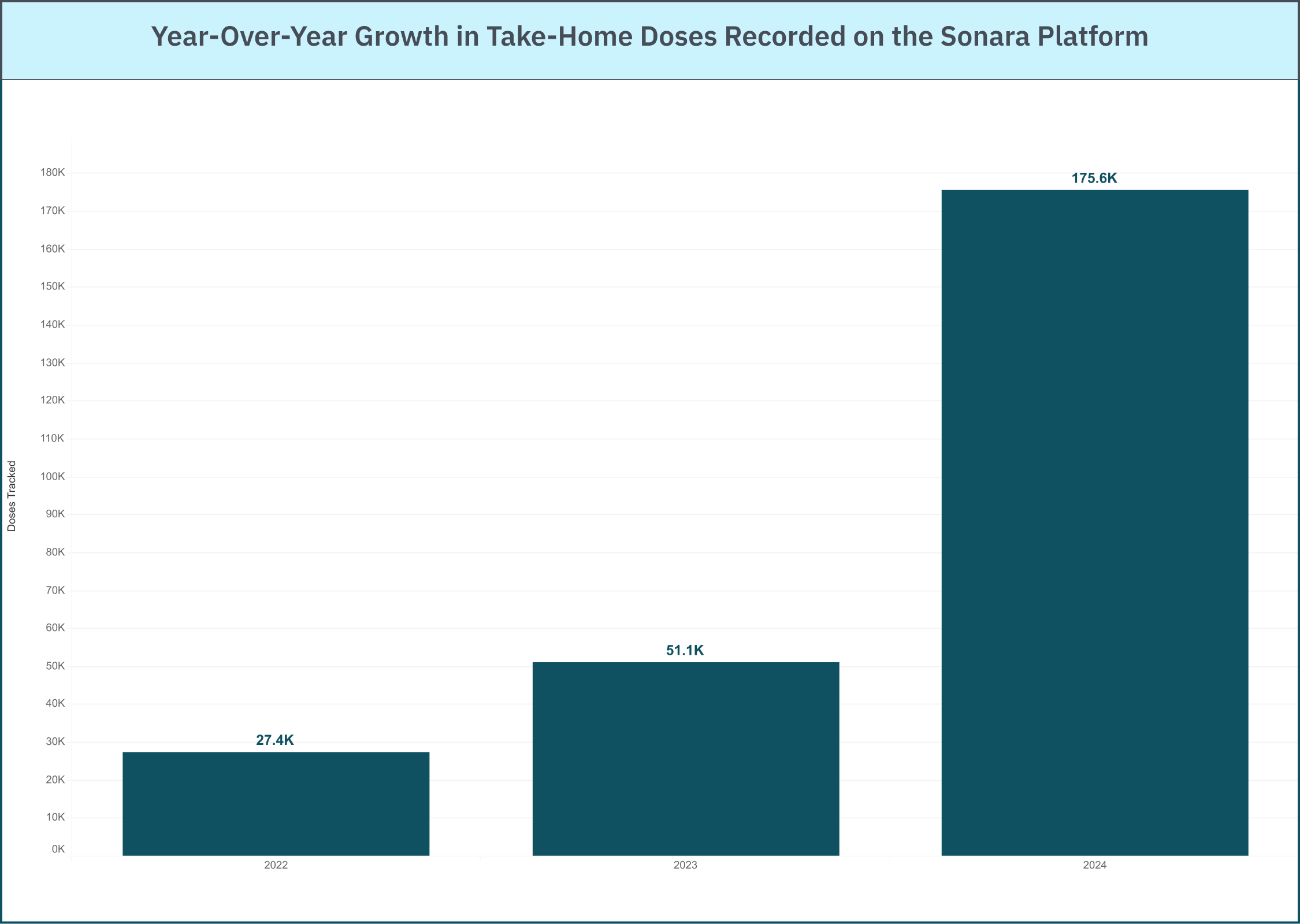
This year, Sonara Health partnered with 36 new treatment centers, expanding our reach into 7 new states. Across these new locations and our existing clinic partners, patients recorded more than 175,000 methadone doses in 2024 through Sonara’s platform.
“Sonara has provided not just increased access for our patients, but has given our providers more security in feeling like they can expand take-homes. It’s just the level of support they needed to feel confident in this step.” - Nicole Ross Regional Director at BHG
This represents a significant number of patients who were able to either: access take-home methadone earlier in their treatment or earn more take-homes at a time. With Sonara, we’re empowering patients to take critical steps toward independence while minimizing risks of diversion or misuse.
As one Sonara patient shared:
“Thank you, for the wonderful opportunity of letting me be part of this Sonara community and for the trust that has been given to us through this program. I do feel that I can now reach achievements that were otherwise unattainable due to always having to retain a consistent presence at my local clinic.”
This year also marked a significant milestone in OUD care as SAMHSA issued its first major updates to OTP regulations in over 50 years.
The 42 CFR Part 8 Final Rule made permanent the expanded take-home flexibilities introduced during the COVID-19 pandemic. It also introduced additional changes focused on removing barriers to care and recommending an individualized approach to OUD treatment.
With these updated guidelines, OTPs now have greater flexibility to issue take-home methadone earlier in treatment, which is shown to improve retention rates and engagement.
Pioneering Research to Transform OUD Care
At Sonara, research is the foundation of our science-informed and equity-led treatment approach. By investing in rigorous clinical studies, we aim to demonstrate the efficacy of our platform, improve our services, and identify opportunities for future innovation. In 2024, we launched two significant research initiatives:
1. Remotely Observed Methadone Evaluation (ROME) Study
Funded by a $2.5 million Small Business Innovation Research (SBIR) grant from the National Institute on Drug Abuse (NIDA), the ROME study seeks to evaluate how Sonara’s platform impacts treatment retention, opioid use, overall costs, and quality of life. Conducted in partnership with Chestnut Health Systems and Family Guidance, Inc., this study also evaluates the effectiveness of our measurement-based care (MBC) tool.
In Phase I of the ROME study, we successfully integrated an MBC tool into our web-based app, demonstrating the feasibility of this approach. Phase II, which began on December 9, 2024, uses a randomized design to build on these findings. The findings will help us refine our platform and strengthen our ability to meet patient and provider needs.
2. Remote Methadone Ingestion System Trial (RMIST)
This study, the RMIST, focuses on the feasibility and efficacy of remote methadone ingestion monitoring, aiming to enhance safety while minimizing the risk of diversion. During this study, feasibility and efficacy are assessed by measuring patient adherence to treatment regimens and usability of the Sonara Virtual Dosing Window™.
These clinical studies bring us closer to our vision of expanding equitable access to life-saving medication through hybrid care and remote observation.
Partnering with State Governments to Pilot New Programs
This year, Sonara partnered with New Jersey to pilot a state-supported remote observation program for take-home methadone. Included in the FY25 budget, this initiative was developed in collaboration with Assemblyman William F. Moen Jr. and the New Jersey Association for the Treatment of Opioid Dependence (NJATOD).
"The inclusion of this pilot program in New Jersey’s budget underscores our commitment to adopting innovative solutions that provide equitable access to treatment for all residents affected by the opioid epidemic." — Assemblyman William F. Moen Jr.
Sonara will implement this program at select OTPs across the state, marking an important step forward in government advocacy for increased methadone access. More than that, it represents a pathway to greater access and advocacy for the communities that need it most.
Looking Ahead to 2025
As we reflect on 2024, we are proud of what we’ve accomplished, but we know our work is far from done. The opioid crisis continues to impact families and communities across the country (and affects Black and Indigenous communities at disproportionate rates), and the need for scalable, equitable solutions has never been greater.
Expand partnerships with OTPs nationwide.
Advance clinical research to strengthen the evidence base for our platform.
Advocate for state and federal policies that increase access to life-saving treatment.
Unlock innovative partnerships with states, payors (particularly Medicaid) and other interested stakeholders to fund our growth initiatives.
Pilot new product features to better support OTPs in delivering patient-centered care from intake through ongoing treatment.
If you are interested in partnering with us in any of these areas, we would love to connect with you. Contact us today.